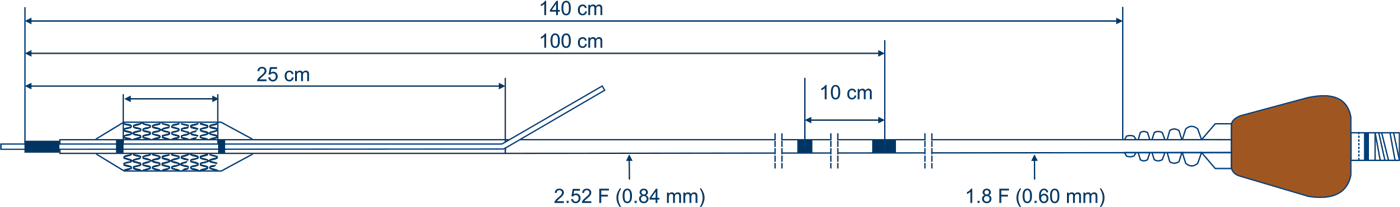
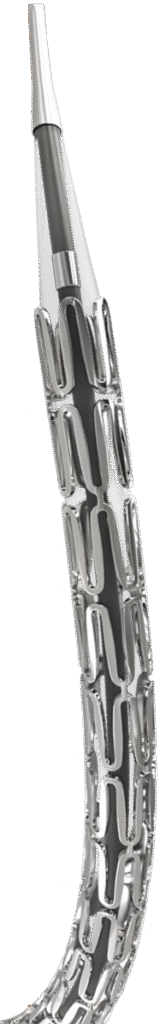
*The above diagram is just an illustration of the product.
Disclaimer: The law restricts these devices to sale by or on the order of a physician. Indications, contradictions, warnings can be found in the product
labelling / IFU supplied with each device. For restricted use only in countries where product is registered with applicable health authorities.
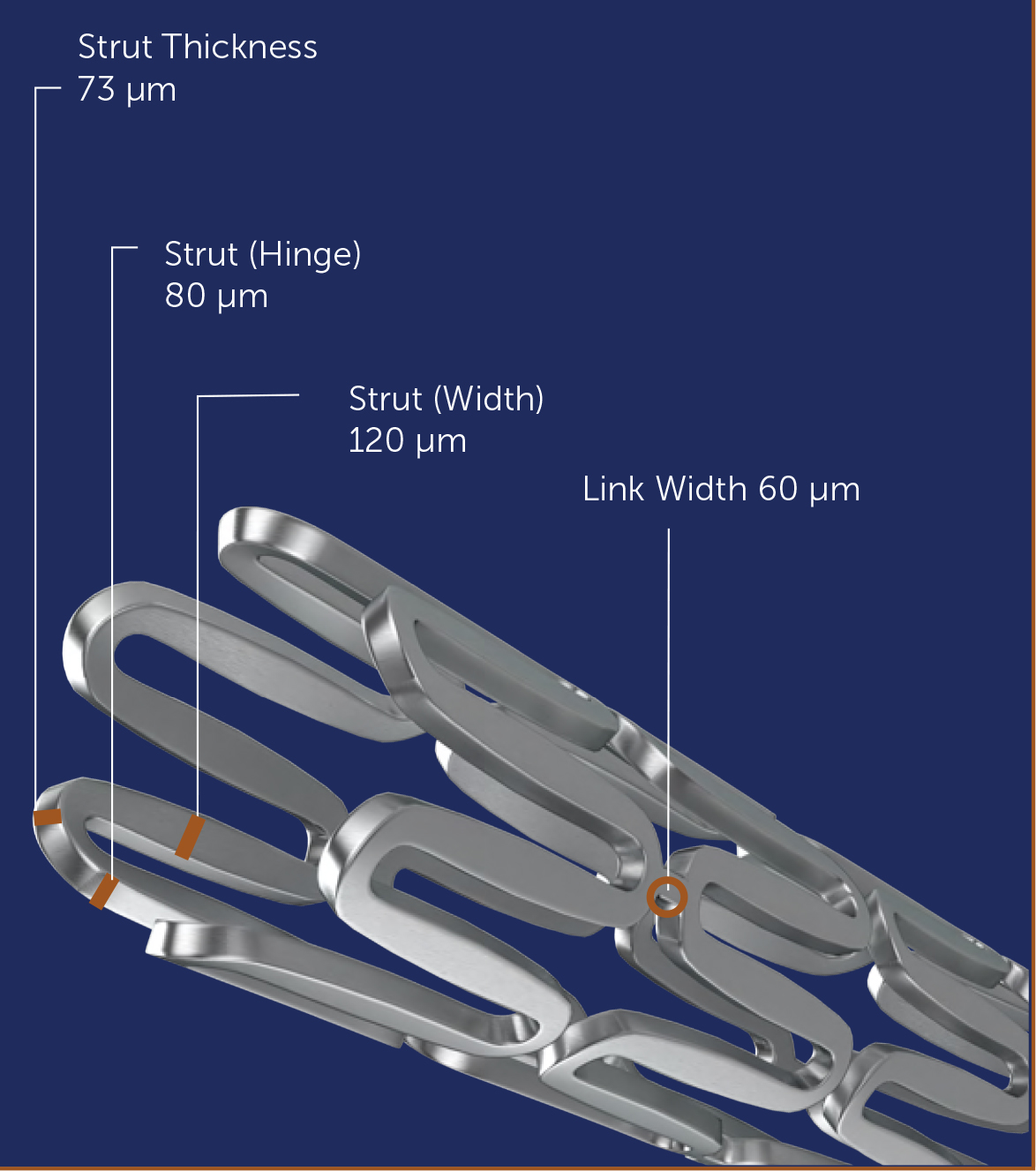
Features
- Bio-Compatible CoCr(L605) Alloy
- Hybrid Cell design for higher strength and flexibility
- Unique strut design for flexibility and larger surface area contact reducing plaque
- prolapse and optimizes metal to artery ratio
- Added Extra Connectors at the Proximal and Distal ends reinforce
- Axial and Radial Strength
IN-VITRO DRUG RELEASE
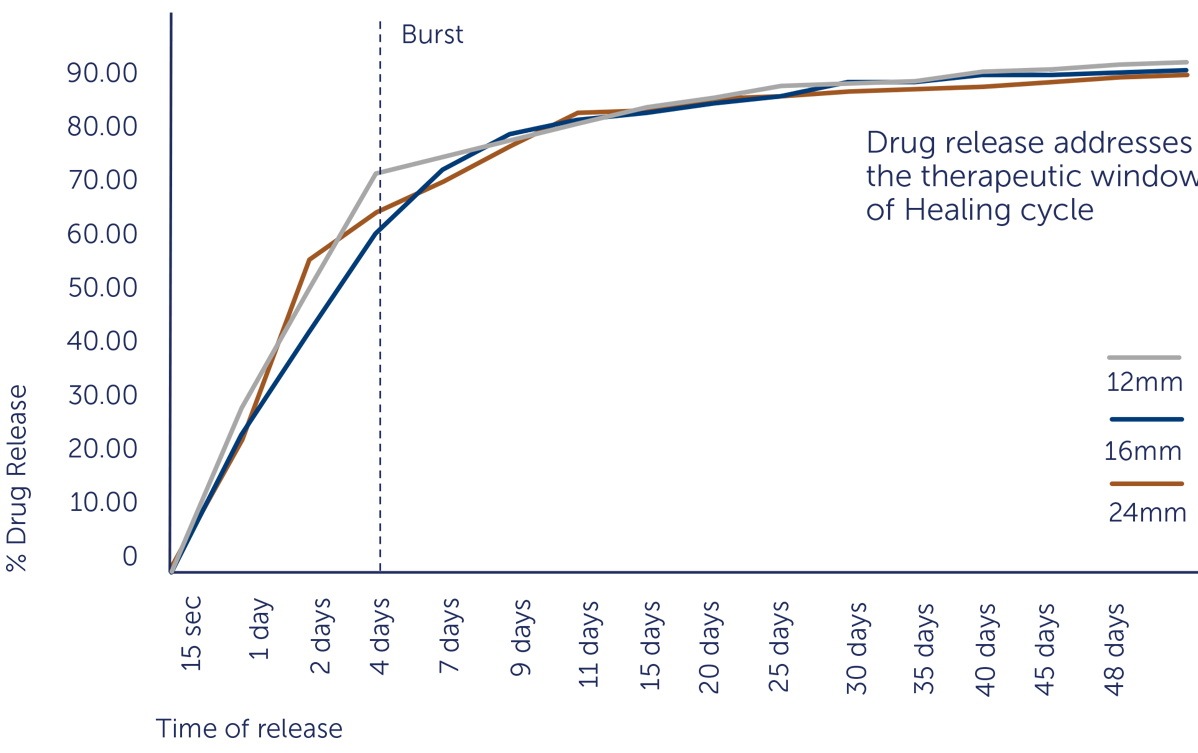
SIROLIMUS ELUTING STENT SYSTEM WITH BIODEGRADABLE POLYMER MATRIX
COATING
Coating of Sirolimus with Biodegradable polymer matrix on the stent facilitate faster endothelialisation
Biodegradable Drug Polymer matrix completely degrades in 6-7 months and converts to BMS thereby reducing late complications
HIGHER POST-DILATATION LIMITS
- Facilitates Sizing flexibility in variable size diameter stenting while using single stent technique in long lesions
- Maintains Metal to Artery ratio across diameters in permissible limits
BETTER SIDE BRANCH AREA ACCESS
Max. Available Side Branch Access Area (CCD*)
- 2.25 – 2.50 mm. Stent – 4.00 mm CCD
- 2.75 – 3.50 mm. Stent – 5.30 mm CCD
- 4.00 – 5.00 mm. Stent – 6.70 mm CCD
*CCD-Circular Cell Diameter